BD Veritor System for Rapid Detection of SARS-CoV-2 and Flu A+B is For use under an Emergency Use Authorization only: https://www.fda.gov/medical-devices/covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-antigen-diagnostic-tests-sars-cov-2 for use at the Point of Care (POC), i.e., i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation
BD Triplex assay requires Veritor™ System firmware version 5.5 or greater; firmware version info is displayed on screen when turning on the analyzer or on the orange sticker if it is attached to the analyzer
Product ships with minimum 60 days dating
This test is authorized for use at the Point of Care (POC), i.e., i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditationtories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high, or waived complexity tests
Laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high, or waived complexity tests
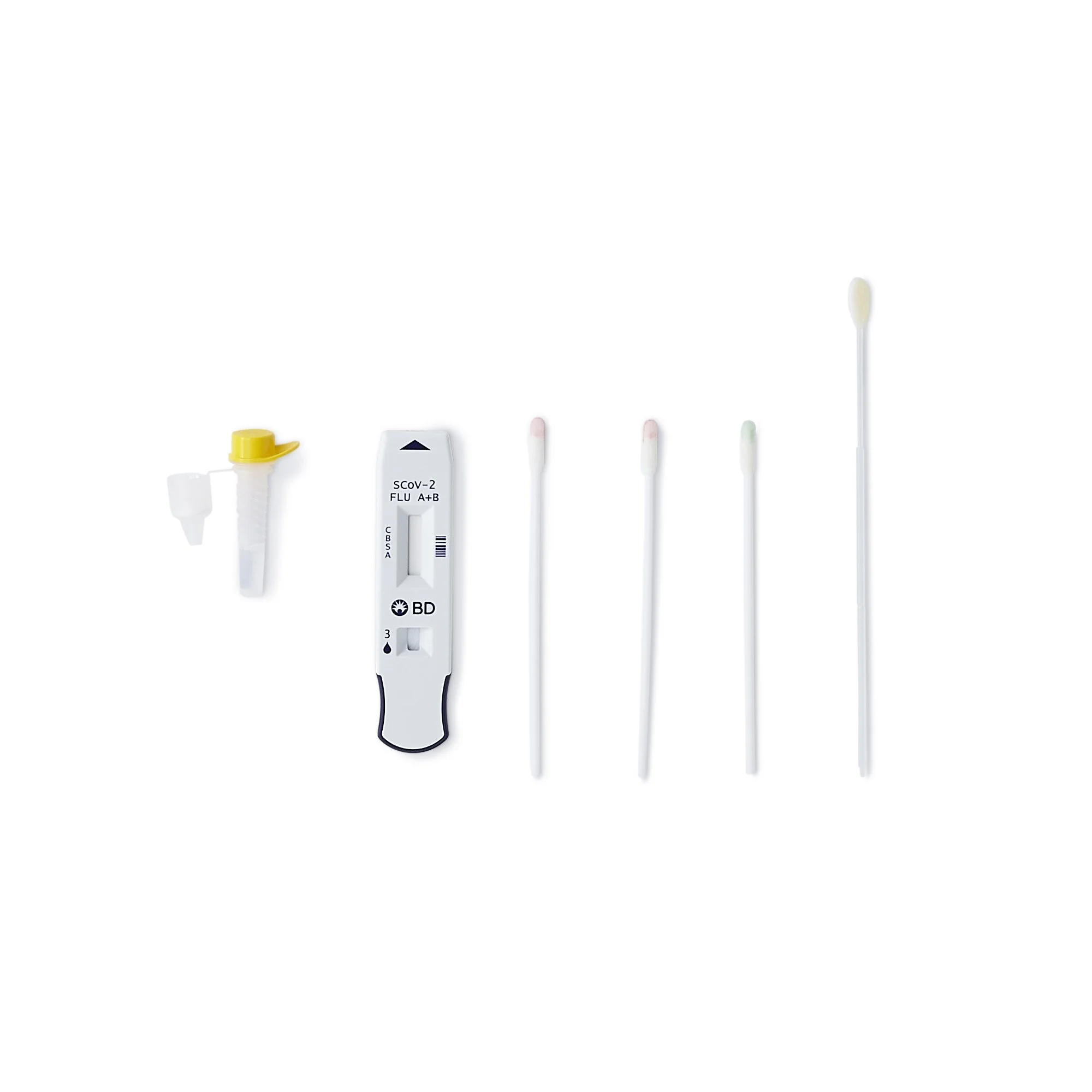
The test is intended to be used with direct nasal swabs and is not validated for use with swabs in viral transport media
Kit configured for testing anterior nasal swab samples freshly collected, processed, and dispensed directly onto assay test device
Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status
Positive results do not rule out bacterial infection or co-infection with other viruses
Laboratories within the United States and its territories are required to report all SARS-CoV-2 results to the appropriate public health authorities
Negative results should be treated as presumptive, do not rule out either Influenza or SARS-CoV-2, and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions
Negative results should be considered in the context of a patient’s recent exposures, history and the presence of clinical signs and symptoms consistent with one of these infections
Negative results for SARS-CoV-2 should be confirmed with a molecular assay, if necessary, for patient management
The BD Veritor System for Rapid Detection of SARS-CoV-2 & Flu A+B is intended for use in point of care settings by trained healthcare professionals or other users specifically instructed in the use of BD Veritor Systems and proper infection control procedures
Time to result: 15 minutes, test device can be read at 15 minutes but no later than 20 minute
Materials Required but not provided: BD Veritor™ Plus Analyzer running firmware v5.50 or later (Cat. No. 256066)
This product has been authorized only for the detection of proteins from SARS-CoV-2, influenza A and influenza B, not for any other viruses or pathogens